


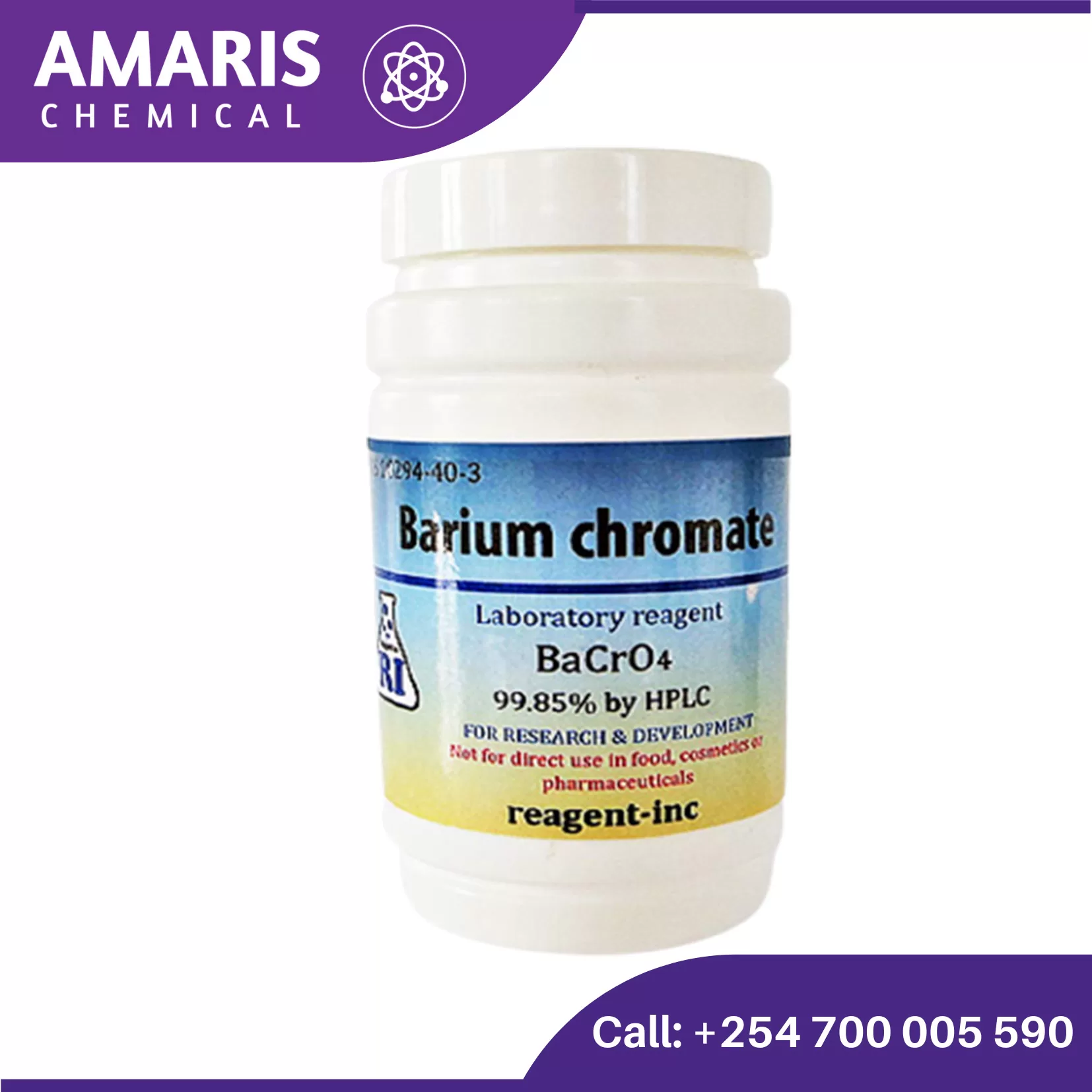
Barium Nitrate 500gm
$2,500.00 Original price was: $2,500.00.$2,300.00Current price is: $2,300.00.
Barium Nitrate is a white crystalline solid which is incombustible yet supports the combustion of burning compounds. If put in fire in large quantities, it may explode.


MAECENAS IACULIS
Vestibulum curae torquent diam diam commodo parturient penatibus nunc dui adipiscing convallis bulum parturient suspendisse parturient a.Parturient in parturient scelerisque nibh lectus quam a natoque adipiscing a vestibulum hendrerit et pharetra fames nunc natoque dui.
ADIPISCING CONVALLIS BULUM
- Vestibulum penatibus nunc dui adipiscing convallis bulum parturient suspendisse.
- Abitur parturient praesent lectus quam a natoque adipiscing a vestibulum hendre.
- Diam parturient dictumst parturient scelerisque nibh lectus.
Scelerisque adipiscing bibendum sem vestibulum et in a a a purus lectus faucibus lobortis tincidunt purus lectus nisl class eros.Condimentum a et ullamcorper dictumst mus et tristique elementum nam inceptos hac parturient scelerisque vestibulum amet elit ut volutpat.
You may also like…
Anhydrous Barium Chloride 500gm
Barium Acetate 500gm
Barium Chromate 250 gm
Barium Hydroxide 8 hydrate
Properties
- Appearance: White crystalline solid.
- Solubility: Moderately soluble in water, forming a strongly alkaline solution.
- Molecular Weight: 171.34 g/mol.
- Density: Approximately 2.18 g/cm³.
- Melting Point: 78 °C (anhydrous form); decomposes in water to form barium oxide and water.
Chemical Behavior
- Basicity: Barium hydroxide is a strong base and dissociates completely in water to form barium ions (Ba2+text{Ba}^{2+}Ba2+) and hydroxide ions (OH−text{OH}^-OH−).
- Reaction with Acids: Reacts with acids to form barium salts and water. For example: Ba(OH)2+2HCl→BaCl2+2H2Otext{Ba(OH)}_2 + 2text{HCl} rightarrow text{BaCl}_2 + 2text{H}_2text{O}Ba(OH)2+2HCl→BaCl2+2H2O
- Hydrate Forms: Often found as the octahydrate (Ba(OH)2⋅8H2Otext{Ba(OH)}_2 cdot 8text{H}_2text{O}Ba(OH)2⋅8H2O).
Barium Sulphate 500g Extra pure
Related products
Acetaldehyde
- Chemical Structure: Acetaldehyde consists of two carbon atoms, one oxygen atom, and four hydrogen atoms. Its structure is CH3CHO, where the carbon atom in the middle is doubly bonded to an oxygen atom and singly bonded to a hydrogen atom and a methyl group (CH3).
- Occurrence: Acetaldehyde can be found naturally in various ripe fruits, coffee, and heated milk. It is also produced by the oxidation of ethanol (alcohol) by enzymes in the liver and other tissues in humans, making it an intermediate product in alcohol metabolism.
Aluminum Fine Powder
Aluminum Potassium Sulphate 500gm
Physical Properties:
- Appearance: Colorless, transparent crystals or white powder.
- Solubility: Soluble in water but insoluble in alcohol.
- Melting Point: Decomposes at high temperatures before melting.
Chemical Properties:
- Molecular Formula: KAl(SO₄)₂·12H₂O
- Molecular Weight: 474.39 g/mol (for the dodecahydrate form)
- Acidity: It is slightly acidic in aqueous solution.




























Reviews
There are no reviews yet.