Bar and gauge apparatus
The bar and gauge apparatus are essential instruments used in laboratories to measure pressure in various systems, whether it be atmospheric pressure or the pressure of gases and liquids in closed systems. The barometer, often referred to as the bar apparatus, is primarily used to measure atmospheric pressure, playing a critical role in experiments involving gas laws and environmental studies. On the other hand, pressure gauges are used to monitor and control the pressure within containers, pipelines, or reactors, ensuring precise pressure management. These devices are vital for maintaining safety and achieving accurate experimental results, particularly in setups involving pressurized gases or liquids
Bar breaking apparatus
The bar breaking apparatus is a precision laboratory instrument designed to evaluate the mechanical strength of materials, specifically their ability to resist breaking under stress. It typically measures tensile strength, flexural strength, or break resistance by applying controlled force to bar-shaped specimens until they fracture. The device is essential in quality control, material testing, and research, offering precise data for materials such as metals, plastics, ceramics, and composites. Its robust design allows for consistent and repeatable testing, ensuring the reliability of the material's mechanical properties.
Barium Hydroxide 8 hydrate
Barium hydroxide, with the chemical formula Ba(OH)2text{Ba(OH)}_2Ba(OH)2, is an inorganic compound that is used in various applications. Here’s a detailed overview:
Properties
- Appearance: White crystalline solid.
- Solubility: Moderately soluble in water, forming a strongly alkaline solution.
- Molecular Weight: 171.34 g/mol.
- Density: Approximately 2.18 g/cm³.
- Melting Point: 78 °C (anhydrous form); decomposes in water to form barium oxide and water.
Chemical Behavior
- Basicity: Barium hydroxide is a strong base and dissociates completely in water to form barium ions (Ba2+text{Ba}^{2+}Ba2+) and hydroxide ions (OH−text{OH}^-OH−).
- Reaction with Acids: Reacts with acids to form barium salts and water. For example: Ba(OH)2+2HCl→BaCl2+2H2Otext{Ba(OH)}_2 + 2text{HCl} rightarrow text{BaCl}_2 + 2text{H}_2text{O}Ba(OH)2+2HCl→BaCl2+2H2O
- Hydrate Forms: Often found as the octahydrate (Ba(OH)2⋅8H2Otext{Ba(OH)}_2 cdot 8text{H}_2text{O}Ba(OH)2⋅8H2O).
Barium Nitrate 500gm
Barium Sulphate 500g Extra pure
Barium sulphate is an inorganic compound with the chemical formula BaSO4. It is a white crystalline solid that is insoluble in water, non-toxic, and chemically inert. Barium sulfate is commonly used in various industries and applications, including medical imaging (as a contrast agent for X-ray and CT scans), in paints, coatings, plastics, rubber, and as a filler in various products due to its high density and opacity. Barium sulfate is a white, odorless, and non-toxic crystalline compound with the chemical formula BaSO4, widely used in medical imaging and various industrial applications for its insolubility in water and inert properties.
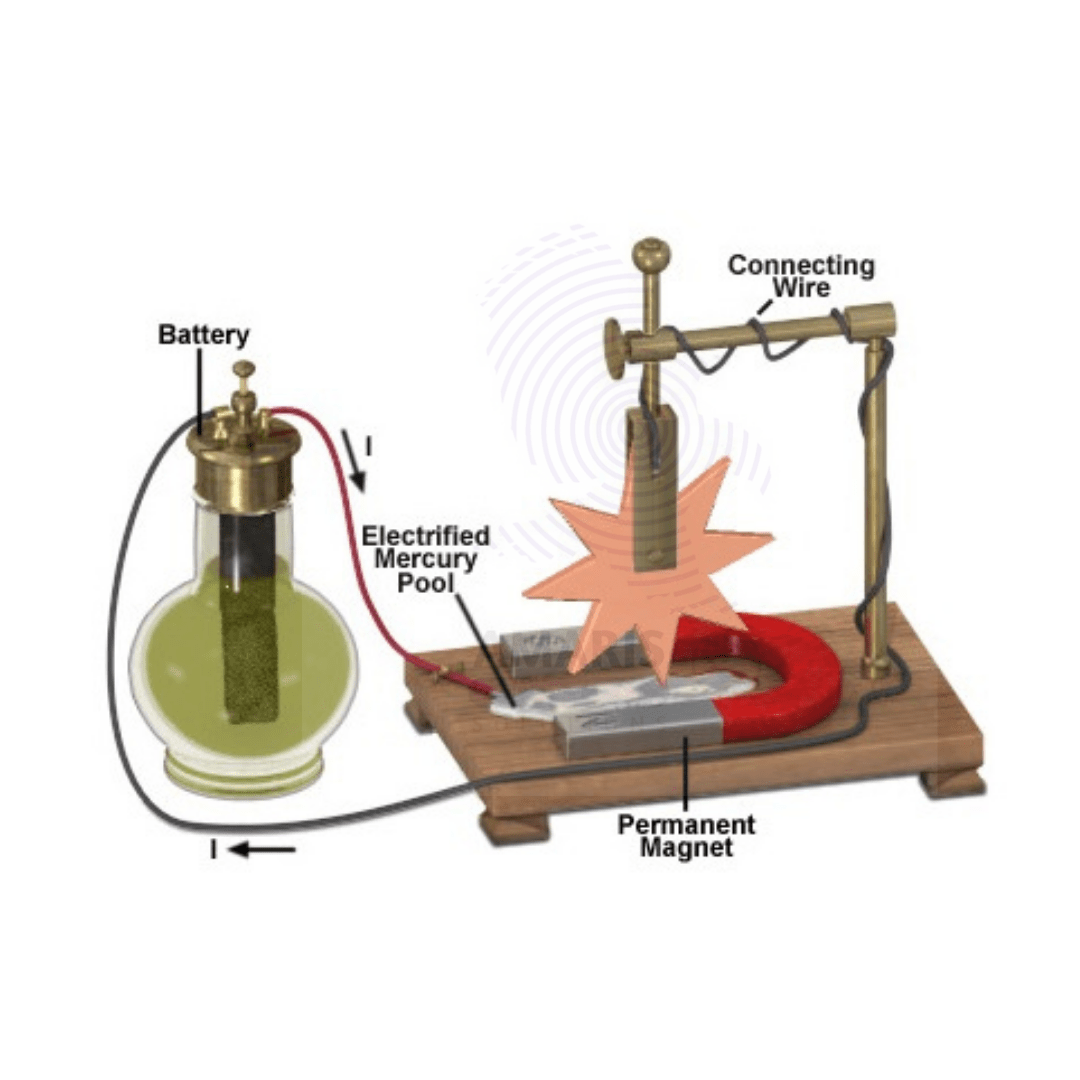
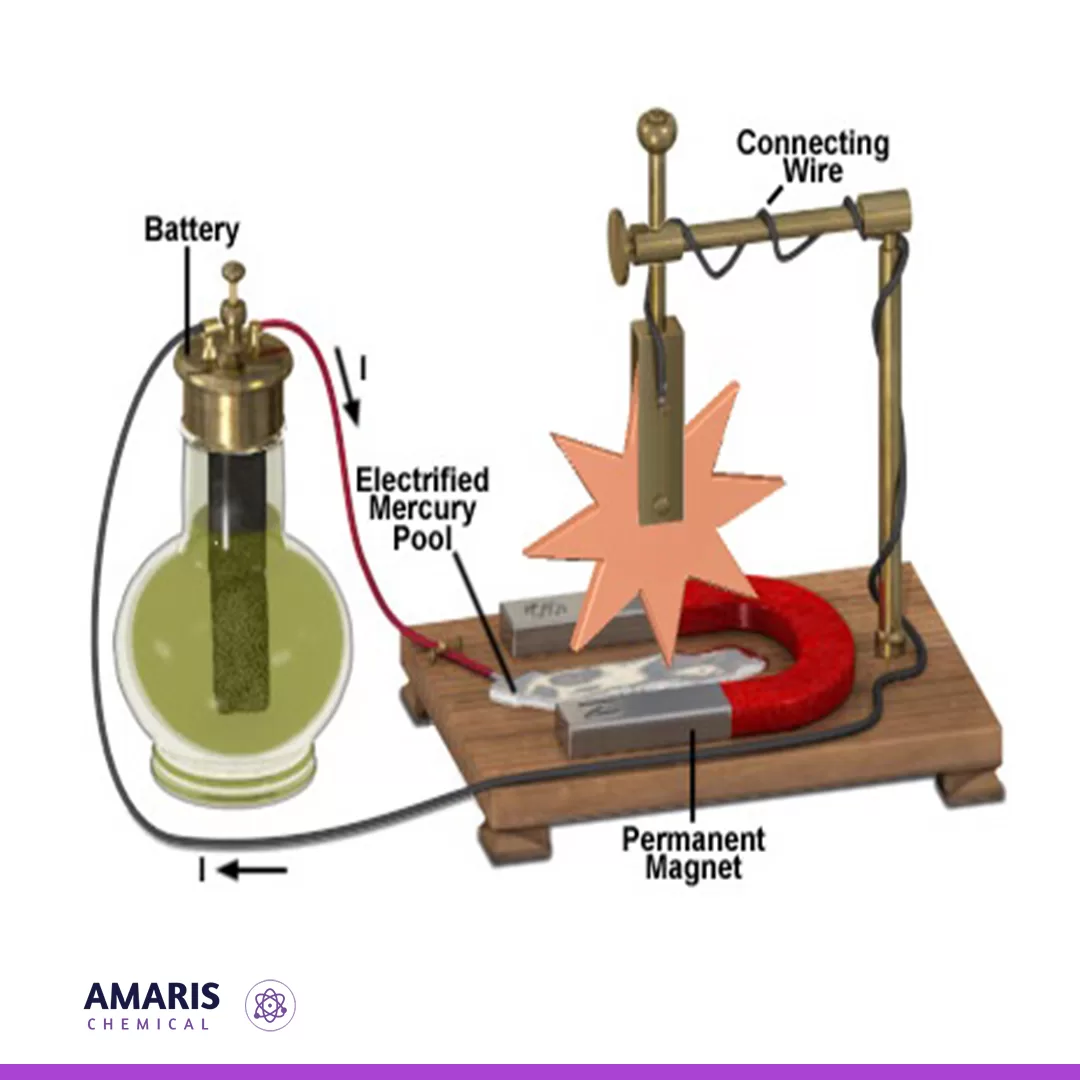
Barlows wheel apparatus
Barlow's wheel, named after the English mathematician and physicist Peter Barlow, is an early demonstration of electromagnetic induction. It consists of a simple apparatus used to generate electricity by rotating a copper disc between the poles of a magnet. When the disc spins, it cuts across the magnetic field lines, inducing an electric current in the disc due to Faraday's law of electromagnetic induction.
The apparatus typically consists of a horizontal axle with a copper disc mounted on it, positioned between the poles of a magnet. The copper disc is connected to a circuit, and when it rotates, an electromotive force (emf) is induced in the disc, causing electric current to flow through the circuit.
Barlow's wheel is a classic demonstration in physics education to illustrate the principles of electromagnetic induction and the generation of electric current. It played a significant role in the development of electrical machinery and the understanding of electromagnetism.
Barometer tubes
A barometer tube is a long, sealed glass or metal tube used to measure atmospheric pressure. It is typically filled with a liquid, such as mercury, with one end sealed and the other submerged in a reservoir of the same liquid. The atmospheric pressure pushes on the reservoir, causing the liquid level in the tube to rise or fall. The height of the liquid column reflects the current atmospheric pressure, allowing for precise readings. Barometer tubes are commonly used in meteorology, physics, and laboratory experiments to observe pressure changes and calibrate instruments.