Great things are on the horizon
Something big is brewing! Our store is in the works and will be launching soon!
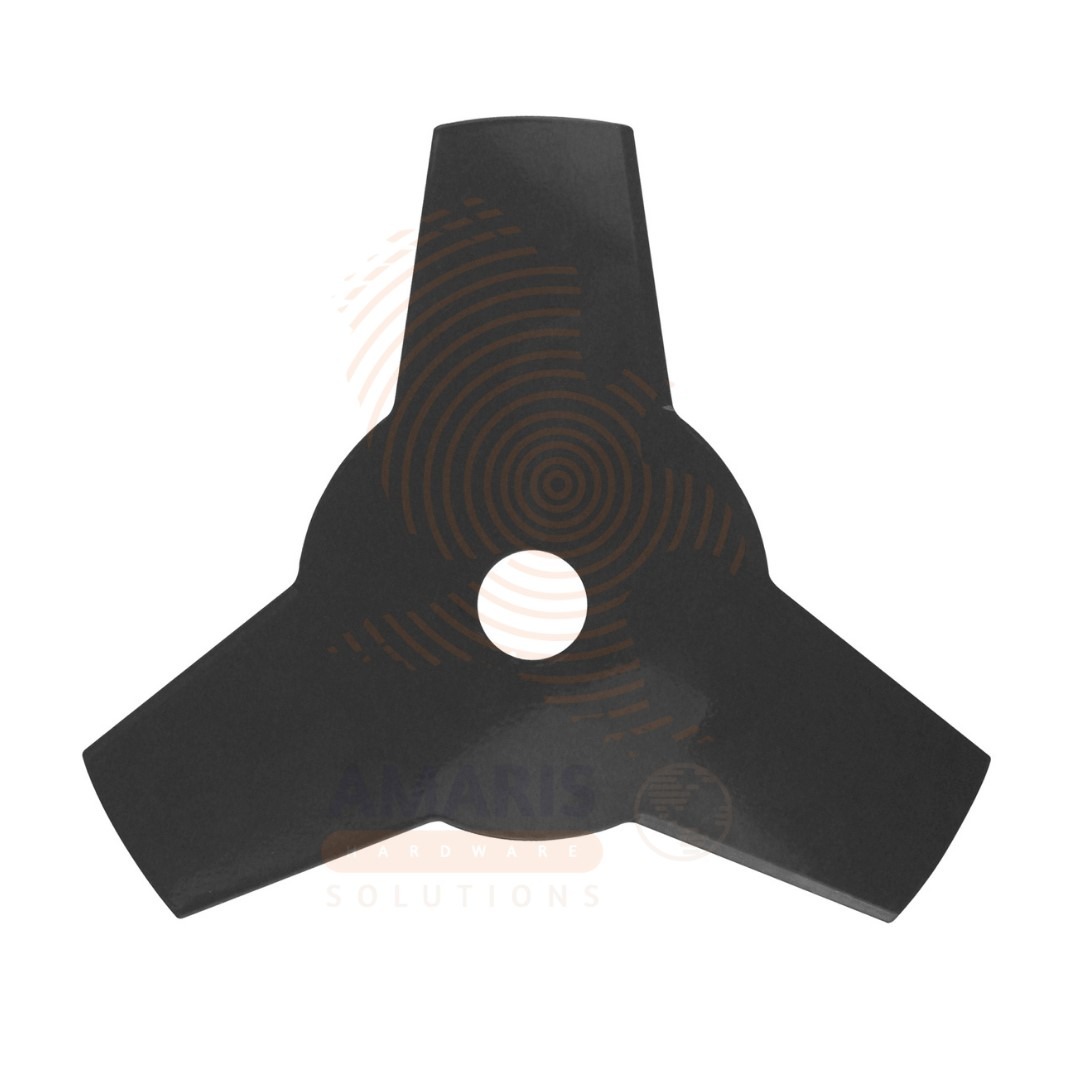 Bush Cutter Blade (12 pcs)
Bush Cutter Blade (12 pcs)
 Heavy Duty Pruner 8.5 (12 pcs)
Heavy Duty Pruner 8.5 (12 pcs)
 Lawn Saw Blade (12 pcs)
Lawn Saw Blade (12 pcs)
 Pump Sprayer 2L (12 pcs)
Pump Sprayer 2L (12 pcs)
Products variations colors and images without any additional plugins.
Something big is brewing! Our store is in the works and will be launching soon!
No account yet?
Create an AccountWill be used in accordance with our Privacy Policy