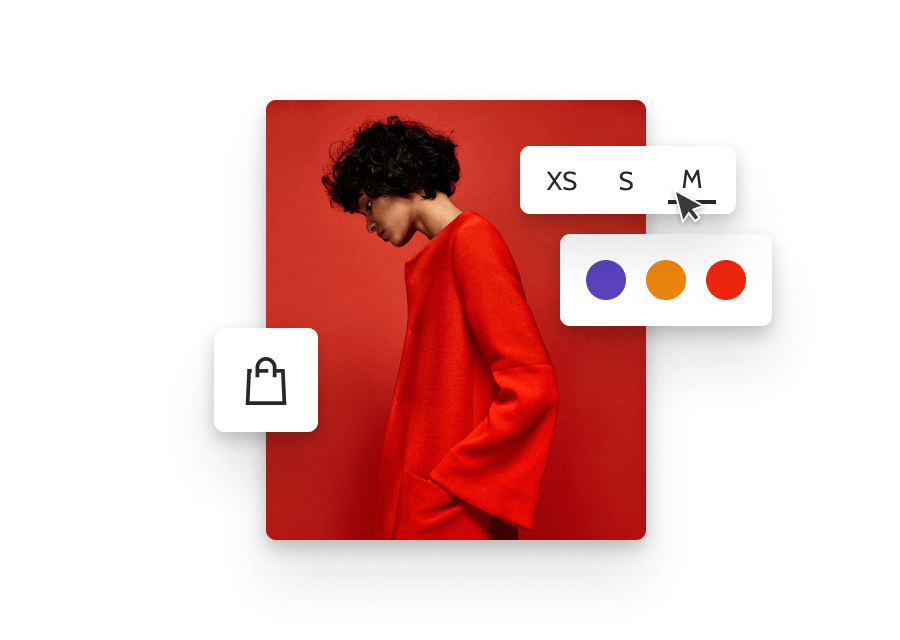
Magnesium oxide 25kg
$12,000.00 Original price was: $12,000.00.$1,100.00Current price is: $1,100.00.
Magnesium oxide is a chemical compound consisting of magnesium (Mg) and oxygen (O). It is commonly known as magnesia or periclase. Here are some key points about magnesium oxide:
- Chemical Formula: The chemical formula for magnesium oxide is MgO.
- Physical Properties:
- It is a white solid at room temperature.
- Magnesium oxide has a high melting point of 2,852°C (5,166°F), making it useful in high-temperature applications.
- It is insoluble in water.
- Formation: Magnesium oxide is typically formed by the calcination (heating) of magnesium carbonate or magnesium hydroxide.
Magnesium oxide
-
- Industrial Applications: It is widely used in various industrial processes, such as in the production of ceramics, refractory materials, and in the manufacture of magnesium salts.
- Medical: Magnesium oxide is used as an antacid and a mineral supplement to treat magnesium deficiency.
- Environmental: It can be used in environmental applications for flue gas desulfurization (removal of sulfur dioxide from exhaust gases).
- Health and Safety: While magnesium oxide is generally considered safe, excessive consumption can lead to magnesium toxicity, causing symptoms like diarrhea and abdominal pain.
- Natural Occurrence: Magnesium oxide occurs naturally as the mineral periclase, which is found in metamorphic rocks.


MAECENAS IACULIS
Vestibulum curae torquent diam diam commodo parturient penatibus nunc dui adipiscing convallis bulum parturient suspendisse parturient a.Parturient in parturient scelerisque nibh lectus quam a natoque adipiscing a vestibulum hendrerit et pharetra fames nunc natoque dui.
ADIPISCING CONVALLIS BULUM
- Vestibulum penatibus nunc dui adipiscing convallis bulum parturient suspendisse.
- Abitur parturient praesent lectus quam a natoque adipiscing a vestibulum hendre.
- Diam parturient dictumst parturient scelerisque nibh lectus.
Scelerisque adipiscing bibendum sem vestibulum et in a a a purus lectus faucibus lobortis tincidunt purus lectus nisl class eros.Condimentum a et ullamcorper dictumst mus et tristique elementum nam inceptos hac parturient scelerisque vestibulum amet elit ut volutpat.