
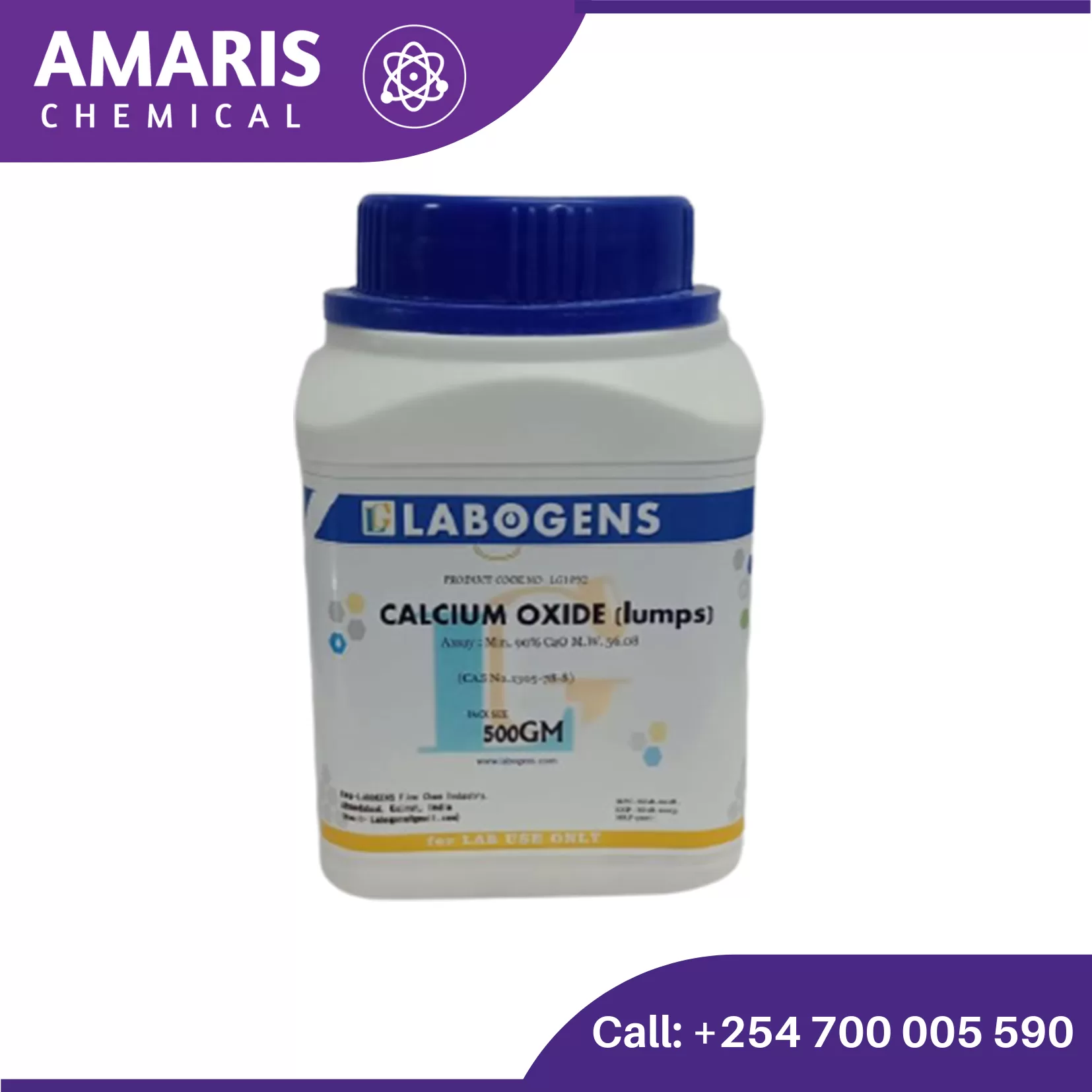
Calcium Sulphate Dihydrate 500gm
$700.00 Original price was: $700.00.$500.00Current price is: $500.00.
Calcium sulfate dihydrate, chemically represented as CaSO4·2H2O, appears as a fine, white to off-white powder. It is odorless and typically occurs as a crystalline solid. In its natural form, it is known as gypsum, which can be found in various geological formations. When heated, gypsum loses its water of crystallization, transforming into calcium sulfate hemihydrate (CaSO4·0.5H2O), commonly known as plaster of Paris, which has important applications in construction and art.
Uses of Calcium Sulphate Dihydrate
Desiccant:
It is used as a drying agent to absorb moisture in desiccators and storage areas.
Chemical Reagent:
It serves as a source of calcium ions and sulfate ions in various chemical reactions and precipitation processes.
Cement and Plaster:
Gypsum is used in the preparation of dental and impression plasters, as well as in the formulation of certain types of cements.
pH Buffer:
It can be used in pH adjustment and buffer solutions due to its ability to provide sulfate ions which can influence pH.
Education and Demonstration:
Gypsum is often used in educational settings for experiments and demonstrations due to its solubility properties and its role in various chemical processes.


MAECENAS IACULIS
Vestibulum curae torquent diam diam commodo parturient penatibus nunc dui adipiscing convallis bulum parturient suspendisse parturient a.Parturient in parturient scelerisque nibh lectus quam a natoque adipiscing a vestibulum hendrerit et pharetra fames nunc natoque dui.
ADIPISCING CONVALLIS BULUM
- Vestibulum penatibus nunc dui adipiscing convallis bulum parturient suspendisse.
- Abitur parturient praesent lectus quam a natoque adipiscing a vestibulum hendre.
- Diam parturient dictumst parturient scelerisque nibh lectus.
Scelerisque adipiscing bibendum sem vestibulum et in a a a purus lectus faucibus lobortis tincidunt purus lectus nisl class eros.Condimentum a et ullamcorper dictumst mus et tristique elementum nam inceptos hac parturient scelerisque vestibulum amet elit ut volutpat.
You may also like…
Calcium Hydroxide(Hydrated lime) 25kg
Related products
2 Propanol 2.5 litres (IPA)
Acetaldehyde
- Chemical Structure: Acetaldehyde consists of two carbon atoms, one oxygen atom, and four hydrogen atoms. Its structure is CH3CHO, where the carbon atom in the middle is doubly bonded to an oxygen atom and singly bonded to a hydrogen atom and a methyl group (CH3).
- Occurrence: Acetaldehyde can be found naturally in various ripe fruits, coffee, and heated milk. It is also produced by the oxidation of ethanol (alcohol) by enzymes in the liver and other tissues in humans, making it an intermediate product in alcohol metabolism.



























Reviews
There are no reviews yet.