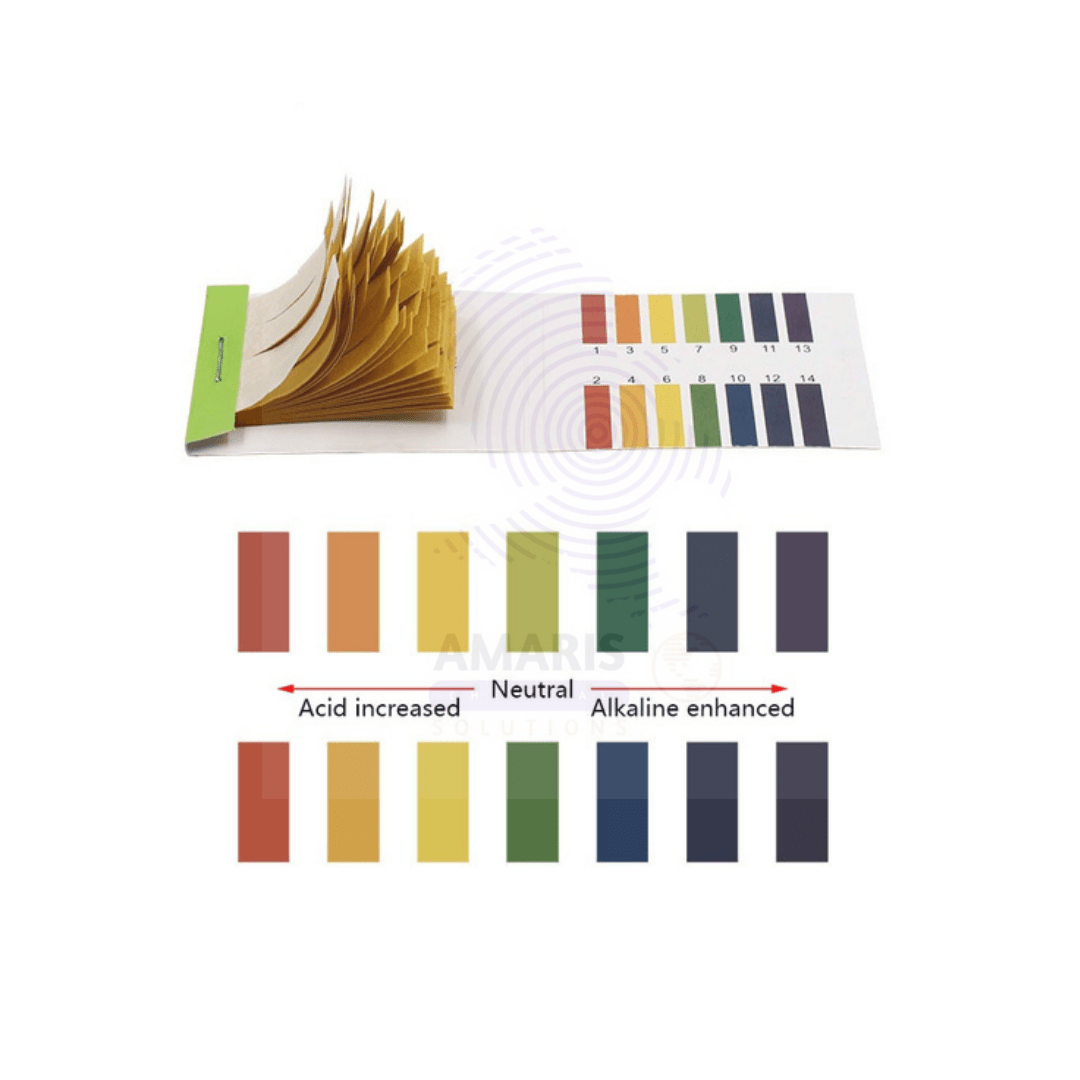
Ph meter strips
pH meter strips, also known as pH test strips or pH paper, are simple, convenient tools used to measure the acidity or alkalinity of a solution. Typically composed of a strip of absorbent paper impregnated with pH-sensitive dyes, they change color based on the pH level of the solution they come into contact with.
Phenol Detached Crystals 500gm
Phenol Detached Crystals" seems to be a specific phrase related to the physical state or appearance of phenol. Phenol, also known as carbolic acid, is an aromatic organic compound with the molecular formula C6H5OHC_6H_5OHC6H5OH. It is a white crystalline solid at room temperature and can be used in the production of plastics, pharmaceuticals, and other chemicals.
If you are referring to "detached crystals," this likely describes a situation where phenol has crystallized out of a solution and the crystals are not adhering to each other or to the container, appearing as separate entities. This can happen under certain conditions, such as when a phenol solution cools down or evaporates, leading to the formation of individual phenol crystals.
Phenol Red 500grams
Phenol Red is a chemical compound with the molecular formula C₁₆H₁₃O₆S. It is a pH indicator dye that changes color in response to pH levels. In acidic conditions (pH below 6.8), it appears yellow, and in alkaline conditions (pH above 8.2), it turns red. This makes it useful in various applications, including biological and environmental sciences, where monitoring pH changes is crucial. Phenol Red is also utilized in medical diagnostics and in the preparation of culture media for microbiology.
Phenolphthalein Solution 500ml
Phenolphthalein is a chemical compound commonly used as a pH indicator in titrations and other analytical chemistry applications. It is a colorless crystalline solid that is often dissolved in alcohol to create a phenolphthalein solution.
Properties of Phenolphthalein Solution
- pH Indicator:
- Acidic Solutions (pH < 8.2): Colorless
- Slightly Basic Solutions (pH 8.2 - 10.0): Pink to fuchsia
- Strongly Basic Solutions (pH > 10.0): Colorless
- Solubility: Phenolphthalein is soluble in alcohol and slightly soluble in water.
Preparation of Phenolphthalein Solution
- Ingredients:
- Phenolphthalein powder
- Ethanol (or another suitable alcohol)
- Distilled water
- Procedure:
- Dissolve 1 gram of phenolphthalein powder in 100 mL of ethanol.
- Dilute the solution with distilled water to make a 1% phenolphthalein solution (typically 100 mL of the ethanol solution to 900 mL of water).
Pin hole camera
A pinhole camera is a simple, lensless optical device that consists of a lightproof box with a tiny hole on one side. Light passes through the pinhole and projects an inverted image of the scene outside onto the opposite surface inside the box. It demonstrates the principles of light traveling in straight lines and image formation, serving as a basic tool for studying optics, image projection, and photography. The pinhole camera provides an infinite depth of field, making all objects, regardless of distance, appear in focus, and is commonly used in educational settings to explore the fundamentals of light behavior.
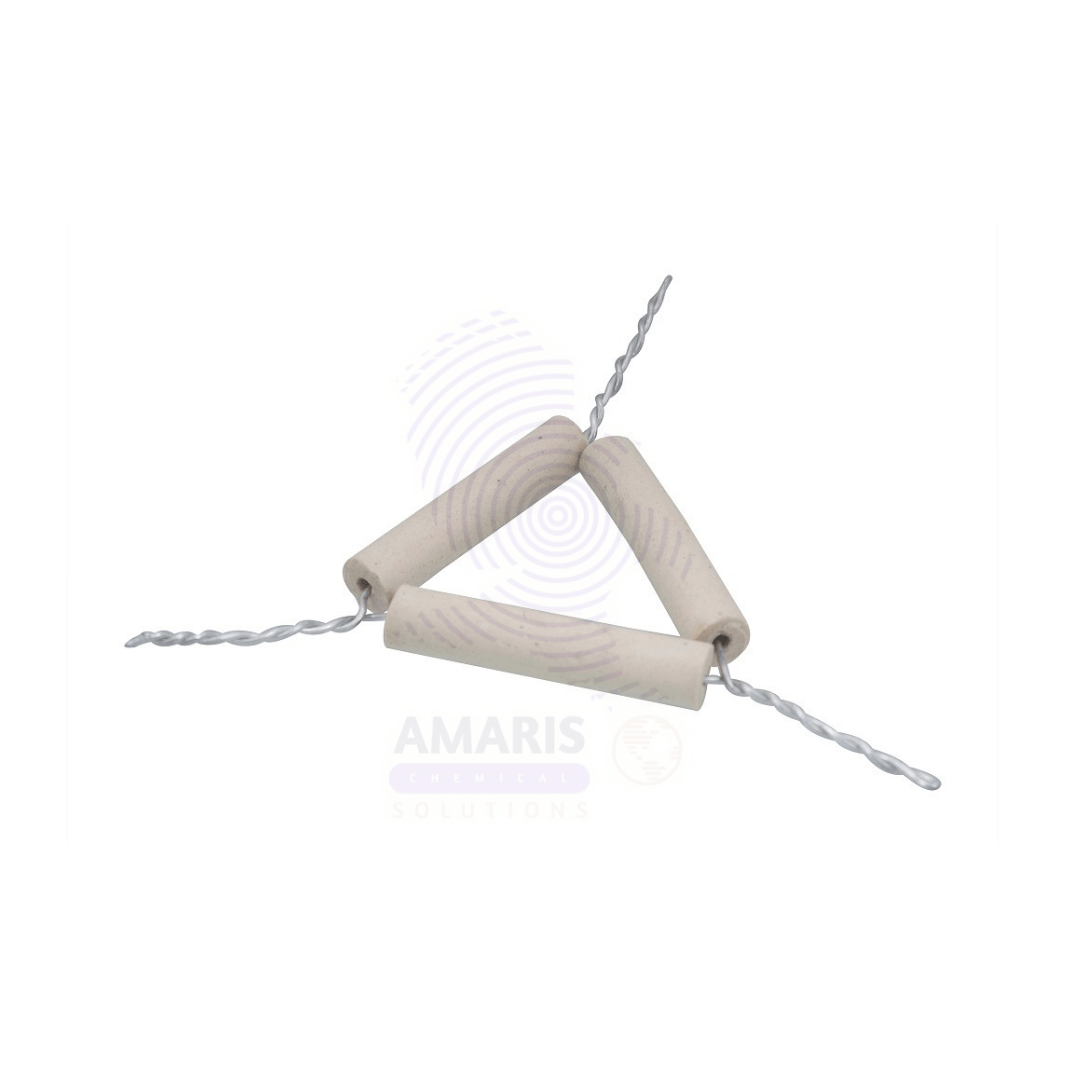
Pipe clay triangles
Pipe clay triangles are laboratory tools made from heat-resistant ceramic material. They are shaped like a triangular frame, designed to support porcelain crucibles or other small containers during heating. The legs of the triangle are typically sturdy and provide stability, allowing for uniform heat distribution when placed over a Bunsen burner or other heat sources. Their ability to withstand high temperatures makes them an essential piece of equipment in various scientific experiments, particularly in chemistry, where precise temperature control is crucial. Overall, pipe clay triangles are durable, reliable, and indispensable for safe heating in laboratory settings.
Pipette Bulb
Pipette bulb, also known as a pipette filler or pipette aid, is a device used in laboratories to draw and dispense liquid accurately using a pipette. It's typically made of rubber or silicone and is attached to the top of a pipette. When squeezed, the bulb creates a vacuum that draws liquid into the pipette. Releasing the bulb dispenses the liquid. This tool helps in precise measurement and transfer of liquids, crucial for various scientific experiments and analyses.